
Management of strictures related to pancreaticobiliary disease continues to be an area of exploration and opportunity for treating both malignant and benign disease. Dilation and stenting of strictures helps patients maintain nutrition often while undergoing neoadjuvent treatment. This web page is intended to provide an venue to share clinical data, case studies and perspectives on biliary stenting from experts in the field to educate other physicians interested in advanced pancreaticobiliary disease management.
Presentation on Stent Management
 Video
Video
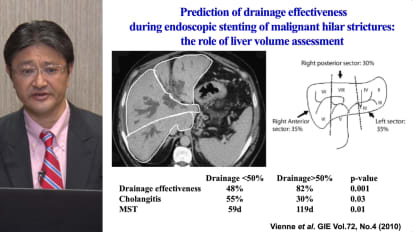
Biliary Metal Stenting Strategies for Malignant Biliary Obstruction, Hiroyuki Isayama, M.D., PhD
Dr. Hiroyuki Isayama from Juntendo University in Tokyo, Japan discusses procedural strategies, situational stent selection and clinical evidence to help manage strictures.
 Video
Video
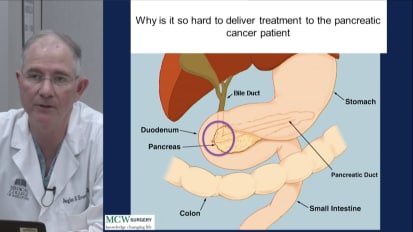
A surgeon’s perspective on the unique challenges of pancreatic cancer. Dr. Evans presents clinical data assessing different approaches to staging, treatment and management.
Set-up and Use Turorials
 Video
Video
Epic™ Biliary Endoscopic Stent System Device Tutorial
This video provides a step-by-step tutorial on the set up and deployment of the Epic Biliary Stent System.
 Video
Video
Using the SpyGlass™ DS System, biliary stones are identified and removed, and targeted biopsies are performed where cholangiocarcinoma is suspected. Once a diagnosis of benign ductal cells is confirmed, a plastic stent is placed.
 Video
Video
WallFlex™ Biliary RX Stent In-service, by Thomas Kowalski, MD
Dr. Kowalski provides a step-by-step demonstration of set-up and deployment of a WallFlex Biliary RX Stent.
Animations
 Video
Video
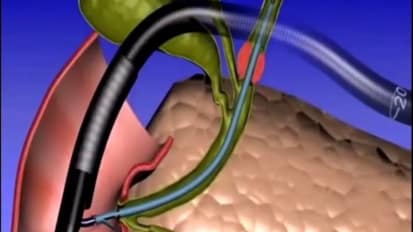
Procedural Animation: WallFlex Biliary RX Stent
This animation includes the steps for placement and deployment of a WallFlex Biliary RX Stent.
 Video
Video
Procedural Animation: Advanix™ Biliary Stent with NaviFlex™ RX Delivery System
This animation highlights the features of the Advanix Biliary Stent with NaviFlex™ RX Delivery System as well as the steps for placement and deployment.
Written Case Studies
Warning: The safety and effectiveness of biliary metal stents for use in the vascular system has not been established.
Results from case studies may vary. Results from case studies are not predictive of results in other case studies.
INDICATIONS FOR USE in the United States:
The WallFlex Biliary RX Fully Covered Stent System RMV is indicated for use in the palliative treatment of biliary strictures produced by malignant neoplasms, relief of malignant biliary obstruction prior to surgery and for indwell up to 12 months in the treatment of benign biliary strictures secondary to chronic pancreatitis.
LIMITATIONS
The sale, distribution, and use of the device are restricted to prescription use inaccordance with 21 CFR §801.109
Contraindications:
• The WallFlex Biliary RX Fully Covered Stent should not be placed in strictures that cannot be dilated enough to pass the delivery system, in a perforated duct, or in very small intrahepatic ducts.
• The WallFlex Biliary RX Fully Covered Stent System RMV should not be used in patients for whom endoscopic techniques are contraindicated.
Warnings: The safety and effectiveness of the stent has not been established for indwell periods exceeding 12 months. The WallFlex Biliary RX Fully Covered Stent System RMV is for single-use only. The safety and effectiveness of the WallFlex Biliary RX Fully Covered Stent System RMV for use in the vascular system has not been established. The safety and effectiveness of the WallFlex Biliary RX Fully Covered Stent System RMV has not been established in the treatment of benign biliary anastomotic strictures in liver transplant patients and benign biliary post abdominal surgery strictures. Testing of overlapped stents has not been conducted. The stent contains nickel, which may cause an allergic reaction in individuals with nickel sensitivity.
PLEASE REFER TO THE LABELING FOR A MORE COMPLETE LIST OF WARNINGS, PRECAUTIONS AND CONTRAINDICATIONS
ACCESS Magazine Clinical Data Update
Patient Education